Modern Periodic Table – The Features, Characteristics & Elements
If you take a closer look at the Modern Periodic Table, you immediately see that it is very organized. You can see the different materials organized just like a big grid.
One of the things that you will also notice is that each one of its elements has a specific location. This location is determined by the element atomic structure.

Just like any grid, the Modern Periodic Table also includes rows and columns. Each one of its rows and columns has specific characteristics. Let’s take a look at Calcium and Magnesium, for example. As you can easily see, they are both located in the same column which means they share some similarities. This also occurs with Calcium and Potassium which share the same row. In addition, Sodium and Magnesium also share similarities since they are in the period, which is the same thing as saying that they have similar electron configurations.
What Are Periods In The Modern Periodic Table?
As we already mentioned, the Modern Periodic Table is similar to a grid and it has rows and columns. Even though there are some holes in the middle of certain rows, you should read the Modern Periodic Table from left to right, as usual.
Each one of the rows of the Modern Periodic Table is called period. If you take a look at the elements in one row, they all have the same number of atomic orbitals.
What Are Groups In The Modern Periodic Table?
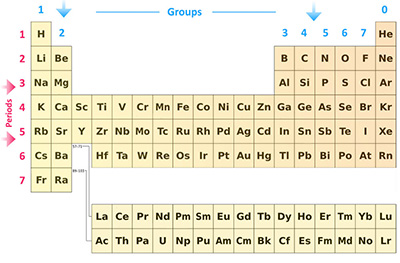
While a row is called a period, each one of the columns in the Modern Periodic Table is called a Group. Just like it happened with the rows or periods, each element of a column or group has the exact same number of electrons in the outer orbital. These are often called valence electrons. In case you don’t know and you’re just starting to learn more about the Modern Periodic Table, these are the electrons that are involved in the chemical bonds with other elements.
If you try to read the Modern Periodic Table, you need to start from left to right again. So, when you take a look at the first group or column, every element that belongs here has one electron in its outer shell. The elements on the second column or group have two electrons in their outer shell. And so on. However, there are some exceptions to this rule that occur with the transition elements.
Special Elements On The Modern Periodic Table
In our opinion, there are two special elements on the Modern Periodic Table that we should take a closer look at:
#1: Hydrogen (H):
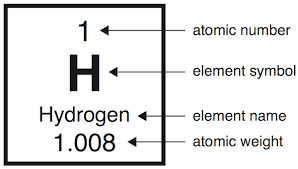
When you look at hydrogen in its neutral form, this element doesn’t have a neutron. It only has one electron and one proton. In what concerns atomic hydrogen, you will need to combine it with other elements to fill its outer shell.
#2: Helium (He):
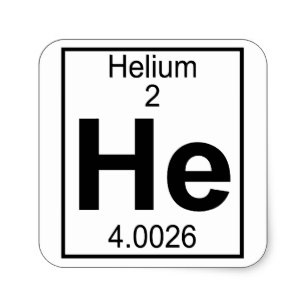
Helium is also a different element. The truth is that this element is incredibly stable even though it only has 2 electrons in its valence shell or outer shell.
One of the things that makes Helium a special element is that it is grouped with the noble gases that usually include 8 electrons in their orbitals.