Electronegativity Chart
Printable charts below ↓
| NUMBER | SYMBOL | ELEMENT | ELECTRONEGATIVITY |
|---|---|---|---|
| 1 | H | Hydrogen | 2.20 |
| 2 | He | Helium | no data |
| 3 | Li | Lithium | 0.98 |
| 4 | Be | Beryllium | 1.57 |
| 5 | B | Boron | 2.04 |
| 6 | C | Carbon | 2.55 |
| 7 | N | Nitrogen | 3.04 |
| 8 | O | Oxygen | 3.44 |
| 9 | F | Fluorine | 3.98 |
| 10 | Ne | Neon | no data |
| 11 | Na | Sodium | 0.93 |
| 12 | Mg | Magnesium | 1.31 |
| 13 | Al | Aluminum | 1.61 |
| 14 | Si | Silicon | 1.90 |
| 15 | P | Phosphorus | 2.19 |
| 16 | S | Sulfur | 2.58 |
| 17 | Cl | Chlorine | 3.16 |
| 18 | Ar | Argon | no data |
| 19 | K | Potassium | 0.82 |
| 20 | Ca | Calcium | 1.00 |
| 21 | Sc | Scandium | 1.36 |
| 22 | Ti | Titanium | 1.54 |
| 23 | V | Vanadium | 1.63 |
| 24 | Cr | Chromium | 1.66 |
| 25 | Mn | Manganese | 1.55 |
| 26 | Fe | Iron | 1.83 |
| 27 | Co | Cobalt | 1.88 |
| 28 | Ni | Nickel | 1.91 |
| 29 | Cu | Copper | 1.90 |
| 30 | Zn | Zinc | 1.65 |
| 31 | Ga | Gallium | 1.81 |
| 32 | Ge | Germanium | 2.01 |
| 33 | As | Arsenic | 2.18 |
| 34 | Se | Selenium | 2.55 |
| 35 | Br | Bromine | 2.96 |
| 36 | Kr | Krypton | 3.00 |
| 37 | Rb | Rubidium | 0.82 |
| 38 | Sr | Strontium | 0.95 |
| 39 | Y | Yttrium | 1.22 |
| 40 | Zr | Zirconium | 1.33 |
| 41 | Nb | Niobium | 1.6 |
| 42 | Mo | Molybdenum | 2.16 |
| 43 | Tc | Technetium | 1.9 |
| 44 | Ru | Ruthenium | 2.2 |
| 45 | Rh | Rhodium | 2.28 |
| 46 | Pd | Palladium | 2.20 |
| 47 | Ag | Silver | 1.93 |
| 48 | Cd | Cadmium | 1.69 |
| 49 | In | Indium | 1.78 |
| 50 | Sn | Tin | 1.96 |
| 51 | Sb | Antimony | 2.05 |
| 52 | Te | Tellurium | 2.1 |
| 53 | I | Iodine | 2.66 |
| 54 | Xe | Xenon | 2.6 |
| 55 | Cs | Cesium | 0.79 |
| 56 | Ba | Barium | 0.89 |
| 57 | La | Lanthanum | 1.10 |
| 58 | Ce | Cerium | 1.12 |
| 59 | Pr | Praseodymium | 1.13 |
| 60 | Nd | Neodymium | 1.14 |
| 61 | Pm | Promethium | 1.13 |
| 62 | Sm | Samarium | 1.17 |
| 63 | Eu | Europium | 1.2 |
| 64 | Gd | Gadolinium | 1.2 |
| 65 | Tb | Terbium | 1.22 |
| 66 | Dy | Dysprosium | 1.23 |
| 67 | Ho | Holmium | 1.24 |
| 68 | Er | Erbium | 1.24 |
| 69 | Tm | Thulium | 1.25 |
| 70 | Yb | Ytterbium | 1.1 |
| 71 | Lu | Lutetium | 1.27 |
| 72 | Hf | Hafnium | 1.3 |
| 73 | Ta | Tantalum | 1.5 |
| 74 | W | Tungsten | 2.36 |
| 75 | Re | Rhenium | 1.9 |
| 76 | Os | Osmium | 2.2 |
| 77 | Ir | Iridium | 2.2 |
| 78 | Pt | Platinum | 2.28 |
| 79 | Au | Gold | 2.54 |
| 80 | Hg | Mercury | 2.00 |
| 81 | Tl | Thallium | 1.62 |
| 82 | Pb | Lead | 2.33 |
| 83 | Bi | Bismuth | 2.02 |
| 84 | Po | Polonium | 2.0 |
| 85 | At | Astatine | 2.2 |
| 86 | Rn | Radon | no data |
| 87 | Fr | Francium | 0.7 |
| 88 | Ra | Radium | 0.89 |
| 89 | Ac | Actinium | 1.1 |
| 90 | Th | Thorium | 1.3 |
| 91 | Pa | Protactinium | 1.5 |
| 92 | U | Uranium | 1.38 |
| 93 | Np | Neptunium | 1.36 |
| 94 | Pu | Plutonium | 1.28 |
| 95 | Am | Americium | 1.3 |
| 96 | Cm | Curium | 1.3 |
| 97 | Bk | Berkelium | 1.3 |
| 98 | Cf | Californium | 1.3 |
| 99 | Es | Einsteinium | 1.3 |
| 100 | Fm | Fermium | 1.3 |
| 101 | Md | Mendelevium | 1.3 |
| 102 | No | Nobelium | 1.3 |
| 103 | Lr | Lawrencium | no data |
| 104 | Rf | Rutherfordium | no data |
| 105 | Db | Dubnium | no data |
| 106 | Sg | Seaborgium | no data |
| 107 | Bh | Bohrium | no data |
| 108 | Hs | Hassium | no data |
| 109 | Mt | Meitnerium | no data |
| 110 | Ds | Darmstadtium | no data |
| 111 | Rg | Roentgenium | no data |
| 112 | Cn | Copernicium | no data |
| 113 | Uut | Ununtrium | no data |
| 114 | Fl | Flerovium | no data |
| 115 | Uup | Ununpentium | no data |
| 116 | Lv | Livermorium | no data |
| 117 | Uus | Ununseptium | no data |
| 118 | Uuo | Ununoctium | no data |
Electronegativity chart 1 PDF to print
Electronegativity chart 2 PDF to print
Simply put, electronegativity is a chemical property that shows how an atom can attract a pair of electrons towards itself. The electronegativity of an atom is influenced by two distinct factors: the distance at which the electrons reside from the charged nucleus and by its atomic number. The electronegativity values run between 0 and 4, and the higher the value, the more the compound or element attracts electrons towards it. This is why it is very helpful to use an electronegativity chart since it can help you visualize.
#1: How Can You Find Electronegativity?
The truth is that each compound or element works in a different way. So, on the table at the top of this page, you can see the different elements as well as their respective electronegativity number.
#2: Using An Electronegativity Chart
As we already mentioned above, electronegativity is a chemical property that shows the tendency that the atom has to attract a share of electrons. Besides, it is affected by two things: the distance of the electrons considering the charged nucleus, and its atomic number.
While there are different scales that you can use such as the Mulliken scale or the Allred-Rochow scale, the one that tends to be most used is the Pauling electronegativity scale.
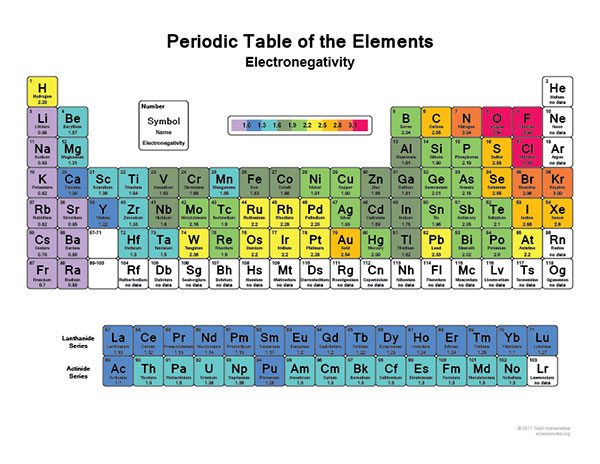
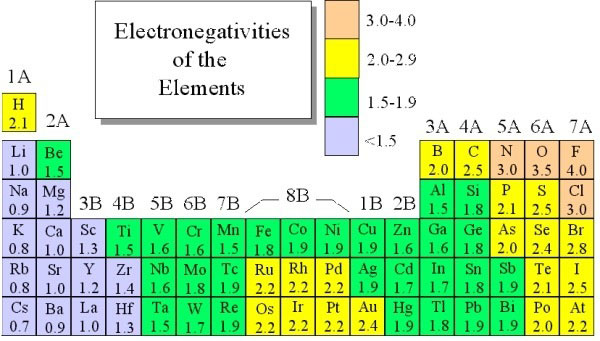
As you can easily see on the image above, the most electronegative atom in the periodic table is fluorine followed immediately by oxygen. On the other hand, the least Electronegative atoms include cesium and francium.
When Linus Pauling started working on electronegativity back in 1932, he took advantage of the Valance Bond Theory. This is how he got the idea that he later proved that there was a relationship between a chemical element with another one. So, it isn’t simply possible to determine the electronegative of an element directly since it depends on the properties of other elements that are related to it in the first place.
The truth is that the Pauling scale helps you determine or calculate the electronegativity of a single element in the periodic table.
It has been shown that the difference of the electronegativity chart will vary with each one of the element’s environment. Additionally, it has also been discovered that atoms that usually have higher electronegativity, tend to attract more electrons from an atom. On the other way around, atoms which have a lower electronegativity, tend to share most of the electrons and, in some cases, they may even lose all their electrons.
The reality is that the electronegativity of an element of the periodic table is influenced by many different factors. These include the atomic charge since the more protons the iota has, the more electrons they will have, and both the number and area that the different electrons exhibit in the nuclear shells. As a particle has more electrons, the farther away from the core this valence of electrons will be. So, they will experience a more negative charge. This is due not only to the fact that they have an expanded separation of the core as well as when the alternate electrons are on the ground within the lower vitality center orbitals, they act as better shields of the valence electrons from the charged core.
According to Pauling, the difference between two bonds (A-B) is a lot stronger than the difference between the same two bonds (A-A and B-B).
- What Happens When Two Atoms That Have The Same Electronegativity Bond Together?
While each one of the atoms, let’s say atom A and atom B, may be forming other bonds with other atoms, we will just consider the relationship between them.
When both the atoms (a and B in this case) are equally electronegative, this means that they will both have the same tendency to attract other pairs of electrons. These will be found between the atoms A and B. In order to find such a bond or relationship, this usually means that A and B need to be the same atom.
- What Happens When The Atom A Is Slightly More Electronegative Than The Atom B?
When this happens, what you will notice is that the atom A will be able to attract more the electron than the atom B. This also means that the end of the bond of the atom A has more electron density which makes it turn slightly negative. In what concerns to the atom B, this one is becoming short on electrons which makes it become slightly more positive.
- What Happens When The Atom A Is A Lot More Electronegative Than The Atom B?
In this case, the pair of electrons is dragged into the end of the bond of atom A. All in all, the atom A loses all the control over its electrons and atom B gains all the control over its electrons. This is how ions are formed.
Returning to the Pauline scale, it is important to have a subjective reference point with a specific goal in order to develop such a scale. In this case, the Hydrogen was chosen as the reference not only because it shapes covalent bonds but also because its electronegativity has been changing throughout the years. While it started as a 3 at 2.1 and was later revised as 7 at 2.20.
#3: Looking At The Most Electronegative Element From The Electronegativity Chart:
When you are looking at the electronegative property of the atom, you realize that every atom shares their electrons to make bonds or relationships with others. The way atoms have to attract electrons is by using their own electronegativity. Ultimately, how many electrons an atom is able to attract is dependent on the atomic number of elements as well as on the distance that is measured by the outer shell of the nucleus.
As we already mentioned above, the most electronegative element is fluorine followed by oxygen.
#4: The Patterns Of Electronegativity In The Periodic Table:
When you are looking at an electronegativity chart and you want to establish some patterns, the best thing you can do is to think about fluorine since it is the most electronegative element in the Periodic Table.
- Looking At Trends In Electronegativity Across A Period
As you are looking at electronegativity across a specific period, you will notice that it increases. If you take a look at the electronegativity chart, you will see that this property moves from the sodium to chlorine. You also need to be aware that you are simply ignoring argon because since it can’t form bonds, it doesn’t have electronegativity.
- Looking At Trends In Electronegativity Down A Group:
When you go down a group, you’ll notice that the electronegativity decreases. The truth is that if it increases up to fluorine, it will decrease as you go down.
So, we can easily conclude that the attraction that a specific bonding pair of electrons gas for a specific nucleus depends on 3 factors:
– the distance from the nucleus
– the number of protons in the nucleus
-the amount of screening by the inner electrons.
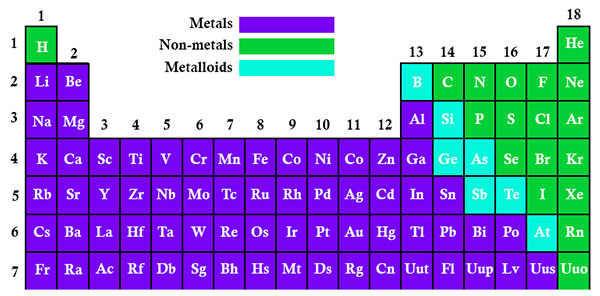
- Why Does The Electronegativity Increases Over A Period:
Just consider sodium which is at the beginning of period 3 and chlorine which is at the end of the same period. Again, ignore argon which is a noble gas. In addition, imagine sodium chlorine as if they are covalently bonded.
As it is easy to understand, both sodium and chlorine have their bonding electrons in the third level. While the electron pair s screened from their nucleus by the 1s, 2s, and 2p electrons, the reality is that the chlorine nucleus has mire 6 protons than the sodium nucleus. So, what you can expect in this situation is that the electron pair is going to be dragged so far towards the chlorine (and away from the sodium) that ions will start to form.
With all this in mind, we can say that the electronegativity increases across a specific period of time because there is an increase on the number of charges on the nucleus. So, ultimately, this ends up by attracting the bonding pair of electrons with more strength.
- Why Does The Electronegativity Decreases As You Go Down A Group?
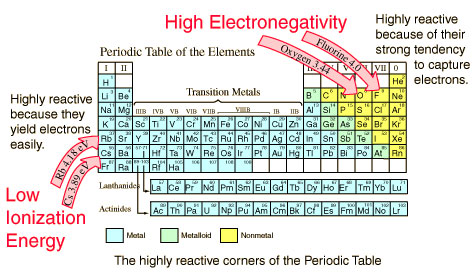
In order to show you this relationship, it’s better to consider hydrogen fluoride and hydrogen chloride.
While in the case of the hydrogen fluoride the bonding pair is shield from its nucleus by only the 1s2 electrons, in the case of the hydrogen chloride it is shield by all the 1s22s22p6 electrons.
In both cases, however, there is exactly the same pull from the center of the fluorine and the chlorine of +7. Nevertheless, while the fluorine has its bonding pair in the second level and the chlorine has its bonding pair in the third level, the one that is closer to the nucleus will have more attraction.
So, we can conclude that as you go down a group (from the third to the second in this particular case), the electronegativity decreases because the bonding pair of electrons is farther away from the attraction of the nucleus.
- Diagonal Relationships In The Periodic Table
Before we even start to discuss the different diagonal relationships in the Periodic Table, it is important to define what a diagonal relationship is in the first place.
The reality is that at the beginning of the periods 2 and 3 of the Periodic Table, you will notice that some elements at the top of each group have some similarities with a specific element on the next group.
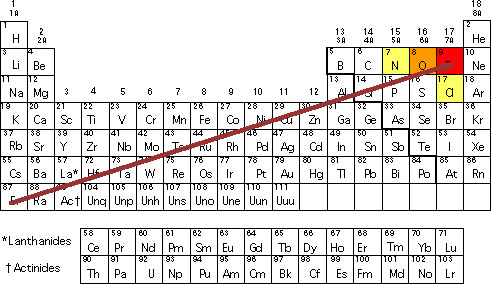
Just consider boron, for example. As you know, boron is a non-metal. However, it shares some properties with silicon. Now, take a look at beryllium. While this element is a part of Group 2, the truth is that it shares some properties with aluminum. Ans the same goes for lithium which is a part of Group 1. Nevertheless, some of its properties differ from the ones of the Group that it belongs to and has some resemblances with magnesium.
If you take a closer look at the Periodic Table and locate the different elements we mentioned, you see that they’re not placed side by side but they are on the same diagonal. This is why we can say that there is a diagonal relationship between these elements.
While there are many different reasons for this to happen, the simplest explanation that we can provide is based on the way that electronegativity varies around the Periodic Table.
As you probably already know, the electronegativity increases over the periodic table. So, let’s take the electronegativities of beryllium and boron, for example:
– Be (beryllium): 1.5
– B (boron): 2.0
As well as the electronegativity falls as you go down the Periodic Table. Just take the electronegativities of boron and aluminum this time:
B (boron): 2.0
Aluminum (Al): 1.5
So, when you take both the electronegativities of the beryllium (Be) and the aluminum (Al), you can see that their values are exactly the same.
So, as you increase from the Group 2 to the Group 3, this increase is offset by the fall when you go from the Group 3 from boron to aluminum. And the same thing happens when you go from lithium (1.0) to magnesium (1.2) as well as when you go from boron (2.0) to silicon (1.8). While in these last two cases the electronegativity values are not exactly the same, they are extremely close.
So, what do these diagonal relationships in the Periodic Table mean?
Simply put, we case say that when there are equal or similar electronegativities between the members of the diagonal pairs, they are likely to form the same type of bonds which will end up affecting their chemistry.
#5: Periodic Table With Electronegativity
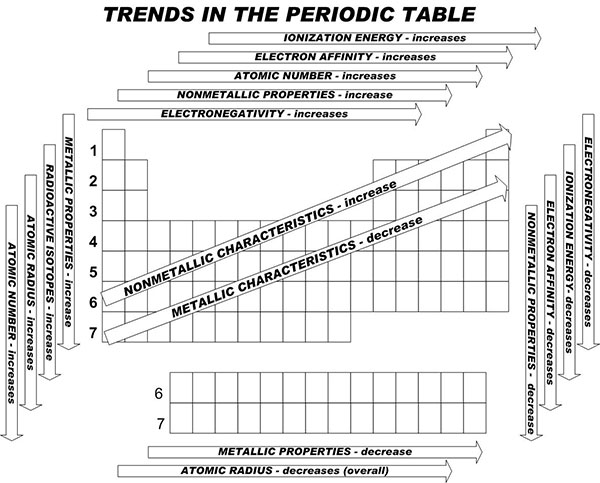
When you take a closer look at a periodic table that includes the different electronegativity numbers for each specific element of the Periodic Table, you’ll notice that when you are moving from the left to the right, the atomic radius of the elements decreases. Nevertheless, the ionization increases as well as the electronegativity.
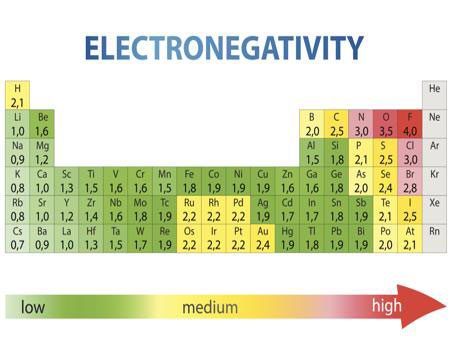
When you now move in a different direction, from the top to bottom, you can also see that the atomic radius tends to increase while the ionization energy decreases. So, electronegativity also decreases.
Another detail that is important to mention is the fact that the noble gas doesn’t have any electronegativity. So, it won’t make any bonds with no other element. And the reason for this to happen is quite simple. The reality is that the outer shell electrons are full with 8 electrons. So, they will have a lower tendency to participate in chemical reactions.
#6: Finding Bonds With Electronegativity:
Sometimes, you may have the need to discover the electronegativity difference between the two atoms.
The reality is that when two atoms are bonded together, the difference between their electronegativity values can give you more details about the qualities of their bond.
In case you need to do this, you just need to take the element with a larger electronegativity and subtract it to the one that has a lower electronegativity.
So, let’s say that you are looking at the HF molecule. You know that hydrogen has an electronegativity of 2.1 while fluorine gas an electronegativity of 4.0. So, in this case, you would need to: 4.0 – 2.1 = 1.9.
Now, you need to learn how you can know more about the value that you just got.
- When the difference of the electronegativities of both elements is below 0.5:
The bond is said to be nonpolar covalent. When this happens, the electrons are shared almost equally between the two elements. In addition, these bonds don’t usually form molecules that have big charge differences. The truth is that these bonds tend to be very difficult to break. One of the best examples we can provide of a nonpolar covalent bond is the molecule O2. Since the two oxygen elements have the same electronegativity, their difference is 0.
- When the difference of the electronegativities of both elements is between 0.5 and 1.6:
When this happens, the bond is named polar covalent. These are the kind of bonds that have more of the electrons at one end than at the other end.
When this occurs, the molecule is a little more negative at the end with the electrons while it is a bit more positive at the other end without them. Due to this imbalance in the charge, this molecule can participate in certain special reactions.
One of the best examples we can provide you of a polar covalent bond is H2O (water). As you probably know, the O (oxygen) is more electronegative than the 2 Hs. So, it will be able to hold the electrons tightly. This will end up affecting the entire molecule since it will be partially negative on the O end and partially positive at the Hs end.
- When the difference of the electronegativity of both elements is between 1.6 and 2.0:
When this happens, you can immediately start searching for a metal. In case there is a metal in the bond that you are evaluating, the bond is also ionic. In case both elements in the bond are non-metal, then you can say that the bond is polar covalent.
When you are looking at the Electronegativity chart of the Periodic Table, you will see that the metals tend to include most of the atoms that are located both on the left and middle of the Periodic Table.
One of the best examples of this difference that we can provide you is the HF. In addition, since neither H or F are metals, you can also conclude that you are now dealing with a polar covalent bond.
- When the difference of the electronegativities of both elements is more than 2.0:
When this happens, the bond is called ionic. In this situation, you will find the electrons sitting at one single end of the bond. One of the characteristics of ionic bonds that is worth to mention is the fact that these atoms tend to react very well with other atoms. They can even be pulled apart by polar atoms.
One of the best examples of an ionic bond that we can show you is the sodium chloride (NaCl). The reality is that the chloride is so electronegative that it is able to pull all the electrons towards it, leaving the sodium with a positive charge.