The Elements Of The Modern Periodic Table
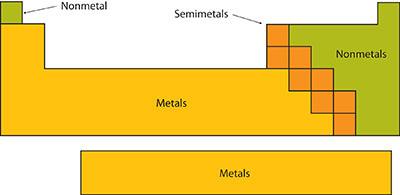
When you are looking at the Modern Periodic Table, it is important to understand that the elements can be divided according to their general properties. So, if you take a closer look at the elements of the modern periodic table, you can easily see that you can divide them into three main categories:

#1: Metals:

Metals can easily be seen in the Periodic Table boxes. After all, they are in the blue, purple, and yellow boxes.
These elements are the majority. Some of the most common examples include gold, silver, lead, and copper. Usually, these elements of the modern periodic table are good conductors of electricity and heat, as well as they are malleable and soft.
One of the things that you may be wondering about is the reason why metals have different colors on their boxes. However, the reason is simple. It refers to the most and less metallic elements. The metals located on the left side and at the bottom of the table are the most metallic elements.
Metals can be divided into Alkali, Alkaline earth, transition or heavy, and rare Earth metals.
#2: NonMetals:

Nonmetal boxes are located above the diagonal line, and they are included in the red, gold, and green boxes on the Periodic Table. Please notice that even though hydrogen is colored with green, it is a metal. However, this is the only exception.
Nonmetals are a very interesting category of elements of the modern periodic table. The truth is that some of these nonmetals exist as gases, others exist as solids, and there is even one that is liquid. Nevertheless, nonmetals share some properties and this is why we are able to form this category.
Within the nonmetals category, you can find fluorine which is the most active nonmetal. On the other hand, you can also find the noble gases as well as the most nonreactive elements.
One of the things that make this category of elements of the modern periodic table so interesting is the fact that they include a lot of compounds that are formed from nitrogen, sulfur, oxygen, hydrogen, and carbon. These are also known as organic chemicals.
A lot of nonmetals are colored. These include elements such as violet-black iodine, red-brown bromine, pale yellow chlorine, yellow-green fluorine, among others.
#3: Metalloids:
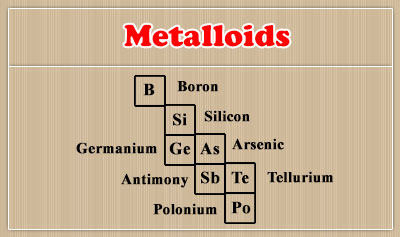
You can easily see metalloids in the Periodic Table when you check the pink boxes and they are in the diagonal.
Metalloids include polonium (Po), tellurium (Te), antimony (Sb), arsenic (As), germanium (Ge), silicon (Si), and boron (B). As you can easily see on the Periodic Table, the metalloids form a stair and they establish the border between the metals and nonmetals categories.
One of the things that you need to know about metalloids is that each one has its own mixture of metals and nonmetals properties. While there are some metalloids that are shiny, others are incredibly luster. Besides, not all of them are able to conduct electricity.