How to Calculate Electronegativity
Before you learn how to calculate electronegativity, it is important to know what electronegativity is.
So, simply put, electronegativity is the process of measuring the strength that an atom has to attract the electrons in a bond. So, as it is easily understandable when an atom has a high electronegativity, it will have more strength to attract electrons. On the other hand, the weaker or the lower the electronegativity of an atom, the weaker the atom is to attract electrons.

One of the reasons why it is important to know how to calculate electronegativity is because the electronegativity values are used to make predictions about how different atoms will behave when they are bonded to each other.
So, how can you calculate electronegativity?
In order to calculate electronegativity, you need to understand that chemical bonds only occur when atoms share electrons. In case you don’t know, a bond occurs when two atoms in a specific molecule are connected to each other. In practice, this means that these atoms share a set of two electrons.
One of the things that you need to understand in order to learn how to calculate electronegativity is that while two atoms can share the same set of electrons, this doesn’t mean that they share them equally. The truth is that if one of the atoms has a higher electronegativity, it will be able to pull the set of electrons near it.
When you are trying to calculate electronegativity, it is important that you have an electronegativity table near you. After all, these table and chart can show you how the different elements are arranged according to their electronegativity.
Here are some considerations that you should know about electronegativity tables:
- As you move to the right of the periodic table, the electronegativity of the atom increases.
- As you move upper on the periodic table, the electronegativity of the atom also increases.
- The atoms with fewer electronegativity can be found down and on the left of the periodic table.
Now that you are well aware of what electronegativity is and why it is useful, let’s see how you can calculate electronegativity.
So, how can you determine the difference of the electronegativity between two atoms?

When the two atoms are bonded together, all you need to do is to calculate the difference between their electronegativity in order to learn more about the qualities of their bond. So, just subtract the atom with a smaller electronegativity from the atom with larger electronegativity.
Let’s say that you were looking to calculate electronegativity of the molecule HF. In this case, you would have:
Electronegativity of Fluorine – Electronegativity of Hydrogen =
= 4.0 – 2.1 = 1.9
After calculating the electronegativity, it is important to analyze the result.
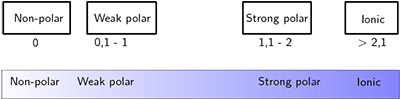
#1: When The Difference Is Below 0.5:
When this happens, we say that the bond is nonpolar covalent. This occurs when the electrons are shared almost equally between the atoms.
#2: When The Difference Is Between 0.5 and 1.6:
When this happens, we say that the bond is polar covalent. This occurs when one of the atoms has a higher electronegativity and is able to attract more electrons near it.
#3: When The Difference Is Between 1.6 And 2.0:
When this happens, you should check for a metal. In case there is at least one metal in the bond, the bond is said to be ionic. In case there aren’t any metals, the bond is said to be polar covalent.
#4: When The Difference Is Over 2.0:
When this happens, we say that the bond is ionic. This occurs when all the electrons are near one single atom.